Patient safety in medical research is critical to ensuring that individuals who volunteer for clinical studies are protected from harm. As medical advancements rely heavily on the participation of human subjects, overseeing these trials under strict research ethics is essential. Recent funding cuts have raised serious concerns regarding IRB oversight, making it increasingly difficult for institutions to maintain the high standards needed for clinical trials safety. When research funding is reduced, particularly federal medical research grants, the support structures designed to safeguard participants may falter, undermining trust in the research process. Therefore, as we navigate these challenges, prioritizing patient safety must remain at the forefront of medical research endeavors.
In the realm of clinical studies, safeguarding the wellbeing of participants is of utmost importance. It is imperative that ethical standards are upheld to ensure the rights and protections of individuals who engage in therapeutic trials. Funding limitations pose a significant threat to the integrity of research oversight, potentially compromising the safety mechanisms that shield volunteers from adverse effects. The role of Institutional Review Boards (IRBs) becomes even more crucial in this context as they navigate the complexities of compliance and ethical practices amidst dwindling resources. As we address the implications of financial constraints in medical exploration, the dialogue surrounding participant welfare must remain a priority.
The Importance of Patient Safety in Medical Research
Patient safety in medical research is a paramount concern for institutional review boards (IRBs) and researchers alike. Given that clinical trials often involve new and experimental treatments, it is essential that comprehensive oversight is maintained throughout the research process. This oversight ensures that ethical standards are upheld, participants are accurately informed, and any potential risks are evaluated and mitigated effectively. Without such measures, individuals may be placed in situations that are harmful, violating the very essence of informed consent and ethical research practices.
Furthermore, patient safety not only protects individual participants but also safeguards public trust in medical research as a whole. Historical examples, like the notorious Tuskegee syphilis study, highlight the catastrophic failures that can arise from a lack of oversight. Presently, the ongoing reliance on IRB oversight ensures that the rights and wellbeing of participants are prioritized while facilitating innovation in therapeutic research, thereby ultimately benefitting society at large.
Impact of Funding Cuts on Research Ethics
Funding cuts pose a significant threat to maintaining ethical standards in medical research. As seen in recent events where a halt in federal research grants disrupted numerous studies, the ramifications are far-reaching. Ethical guidelines that govern clinical trials are typically supported through financial investment, including resources for training IRB personnel and conducting thorough oversight. When funding is reduced, institutions struggle to operate programs that uphold research ethics, which can lead to compromises in participant protection and oversight quality.
In addition, a decrease in financial support can hinder the ability of research institutions to provide adequate resources for thorough IRB review processes or recruit qualified professionals who specialize in ethical compliance. The lack of necessary funding not only jeopardizes the rights and wellbeing of study participants but can also lead to a reduced commitment to uphold ethical research standards. This situation fosters an environment where unethical practices may emerge, undermining the integrity of the research community.
The Role of IRB Oversight in Protecting Participants
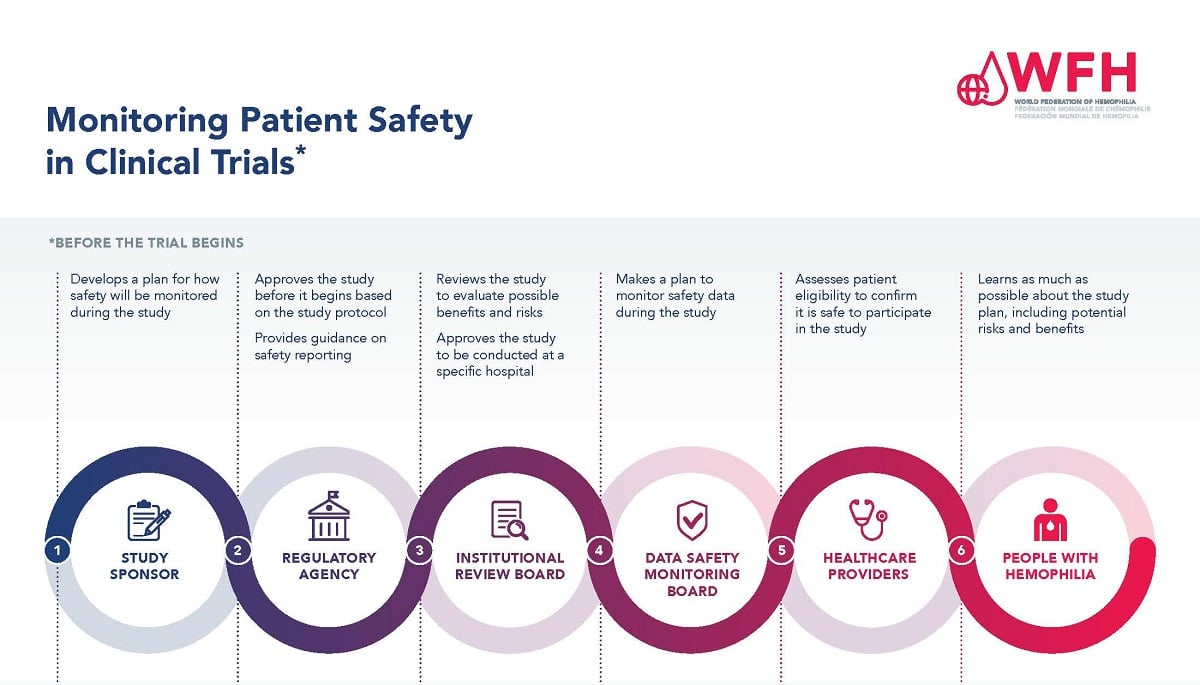
Institutional Review Boards (IRBs) are fundamental in safeguarding participant welfare by ensuring that all research studies undergo scrutiny prior to commencement. IRB oversight includes reviewing research proposals to assess risks, ensure informed consent, and monitor compliance with ethical standards. This thorough review process is critical in clinical trials, as it addresses potential risks and helps to mitigate any adverse effects that may arise from the participation of human subjects.
Moreover, the implementation of a single IRB for multisite studies, as mandated by NIH policy, enhances efficiency and consistency in oversight, ultimately protecting participants across various locations. This unified review process helps streamline approvals while still maintaining the highest ethical standards and participant safety. By reinforcing IRB oversight, research institutions continue to cultivate a culture of safety that benefits both participants and the broader medical research community.
The Historical Context of Research Ethics
The evolution of research ethics is rooted in past transgressions where the rights and dignity of research participants were severely compromised. Incidents such as the Tuskegee syphilis study and unethical experiments during World War II highlighted the dire need for stringent ethical oversight in medical research. These events prompted significant legal and moral reforms, leading to the establishment of diverse regulatory mechanisms, including IRBs, to protect human subjects in research.
Today, the impact of these historical lessons is felt deeply within the medical research industry. Modern-day IRBs are tasked with ensuring that all research adheres to ethical guidelines, not only to prevent past wrongs from recurring but also to foster a climate of trust and transparency. The incorporation of ethical review processes helps researchers maintain accountability and prioritizes the safety and welfare of participants, ensuring that individuals volunteer for studies with a clear understanding and confidence in their protection.
Funding and Resource Allocation in Medical Research
The allocation of funding in medical research is crucial for supporting diverse projects and studies aimed at improving public health. With the current landscape of increased scrutiny and the reallocation of federal funds, researchers face challenges that could compromise the advancement of pivotal studies. The recent funding cuts have raised alarms about interruptions in essential research that relies on government support, undermining the importance of thorough investigations necessary for patient safety.
Moreover, a lack of proper funding directly affects the infrastructure that supports IRBs and other oversight committees. Without adequate financial resources, many research institutions may struggle to recruit and retain qualified personnel whose expertise is essential for maintaining ethical compliance in studies. The result can lead to insufficient review processes and potentially harm the participants involved in clinical trials. As the research community continues to advocate for sustained funding, the emphasis on patient safety and ethical practices must remain at the forefront of institutional priorities.
Enhancing Public Trust in Medical Research
Building and maintaining public trust in medical research is essential for ongoing collaboration and involvement from participants. Trust is often predicated on the transparency and accountability of IRBs, which play a crucial role in ensuring participant safety. A well-functioning system of oversight not only protects those participating in studies but also reinforces the public’s confidence in medical advancements derived from research efforts.
However, in light of funding cuts and potential unethical practices creeping into the research landscape, public skepticism may grow. It’s vital for researchers to engage with communities and demonstrate their commitment to ethical practices and participant safety. By promoting effective communication about the role of IRBs, the importance of informed consent, and the ethical conduct of research, it’s possible to cultivate a resilient trust in the medical research community, benefitting public health initiatives across the nation.
Clinical Trials: Balancing Innovation and Safety
Clinical trials serve as a necessary path for innovation in medical treatments, yet balancing the push for breakthroughs with the imperative of participant safety is critical. As researchers explore new therapies, their primary focus must be on adhering to ethical standards and ensuring that participants are not exposed to undue risks. The careful scrutiny provided by IRBs plays a pivotal role in maintaining this balance, evaluating both the scientific merit of trials and the safety protocols in place to protect participants.
Moreover, thoughtful design and oversight of clinical trials can mitigate risks while still allowing researchers to pursue innovative solutions to medical challenges. This balance is increasingly important in an era where funding cuts may limit the resources available for ensuring these standards. Establishing a collaborative environment where researchers, participants, and IRBs share insights and concerns can facilitate a stronger commitment to safety while fostering the advancements needed in clinical research.
The Future of Ethical Oversight in Research
Looking ahead, the future of ethical oversight in research must adapt to the evolving landscape of medical studies and the challenges posed by funding constraints. As the demand for novel therapies grows, maintaining robust systems for IRB oversight become increasingly vital. A focus on enhancing transparency, communication, and collaboration among stakeholders will be essential for ensuring that research remains ethical and prioritizes participant safety.
Furthermore, as technology continues to advance and influence how research is conducted, regulatory frameworks must also innovate to keep pace. Emphasizing ongoing education and training for IRB members regarding new ethical dilemmas arising from modern medical research will help in addressing the complexities that may emerge. By fostering a proactive approach to ethics in research, the community can ensure the protection of participants and the integrity of scientific inquiry for generations to come.
Collaboration in Multisite Research: A Path to Safety
Collaboration across multiple sites in research is becoming increasingly commonplace, presenting both opportunities and challenges in maintaining participant safety. The streamline review process introduced by the sIRB requirement allows for better efficiency and consistency in how ethical standards are applied across various institutions. This setup can greatly enhance patient safety, as it ensures that all sites involved are following the same guidelines and protocols.
However, the success of such collaborative efforts heavily depends on the availability of resources and funding to adequately support these initiatives. Cutbacks in funding could lead to delays or difficulties in maintaining the high safety standards required. Therefore, it is crucial for institutions to advocate for the necessary financial backing to support these collaborative efforts, fostering an environment where innovation can thrive without compromising the safety and welfare of participants involved in research.
Frequently Asked Questions
What is the role of IRB oversight in patient safety within medical research?
IRB oversight plays a pivotal role in maintaining patient safety in medical research by reviewing research proposals to ensure compliance with ethical standards. This includes evaluating study design, recruitment strategies, informed consent processes, and risk mitigation plans. With rigorous review processes, IRBs protect the rights and welfare of participants, which is essential for maintaining ethical standards in research.
How do funding cuts impact patient safety in clinical trials?
Funding cuts can significantly jeopardize patient safety in clinical trials by halting ongoing studies and preventing the initiation of new research. When federal grants are reduced, institutions face challenges in conducting necessary oversight through IRBs, compromising the safeguards designed to protect participants. Disruptions caused by funding decreases can lead to delays in research that could contribute valuable new therapies, ultimately risking participants’ health.
Why is research ethics critical for patient safety in medical research?
Research ethics is critical for patient safety in medical research as it establishes the framework for ethical conduct during studies. Adhering to ethical guidelines ensures that researchers prioritize the safety, well-being, and informed consent of participants. Robust ethical oversight, including IRB involvement, protects against potential harms and reinforces public trust in the research enterprise, fostering a safer environment for patient participation.
What are the implications of IRB oversight for research involving vulnerable populations?
IRB oversight is particularly significant for research involving vulnerable populations, as it ensures that additional safeguards are in place to protect these individuals from potential exploitation or harm. Vulnerable groups may include children, economically disadvantaged individuals, or those with cognitive impairments. Enhanced IRB scrutiny helps to ensure that their rights and welfare are prioritized, mitigating risks while allowing for ethical engagement in medical research.
How does SMART IRB contribute to improving patient safety in multisite clinical trials?
SMART IRB standardizes the review process for multisite clinical trials, simplifying regulatory oversight and ensuring consistent patient safety standards across all participating sites. By allowing a single IRB to oversee multiple locations, SMART IRB reduces redundancy, accelerates approval timelines, and enhances the focus on safeguarding participants. This streamlined process supports comprehensive oversight, ultimately fostering a safer research environment.
What actions can be taken to maintain patient safety amidst funding cuts in medical research?
To maintain patient safety amidst funding cuts, it is crucial for institutions to advocate for the preservation of research funding and explore alternative funding mechanisms, such as private grants or partnerships. Additionally, enhancing collaboration between research institutions and community stakeholders can help prioritize patient safety. Training IRB members on adaptive strategies to safeguard participants while navigating funding constraints is also essential.
| Key Point | Details |
|---|---|
| Funding Cuts | Federal funding cuts, particularly over $2 billion to Harvard, disrupt crucial research oversight. |
| IRB’s Role | Institutional Review Boards (IRBs) review research to ensure participants’ rights and safety are protected. |
| Research Oversight | Single IRBs (sIRBs) have streamlined oversight processes, ensuring consistent evaluation across multiple research sites. |
| History of Ethics | Historical abuses highlight the need for rigorous ethical oversight in human research. |
| Impacts of Stopping Research | Halting ongoing studies can cause harm to participants and breed public distrust in medical research. |
Summary
Patient safety in medical research is paramount, and recent funding cuts threaten the integrity of research oversight needed to protect participants. With the halt in federal funding, critical institutions face obstacles that hinder their capability to monitor research ethically and effectively. The implications of these funding disruptions extend beyond immediate research activities; they jeopardize the safety and rights of the individuals participating in these studies and may create lasting impacts on public trust in clinical research. It is essential to prioritize and secure adequate funding for these oversight mechanisms to ensure that patient safety remains a top priority in medical research.