Alzheimer’s research stands at the forefront of scientific inquiry as we strive to unravel the complex mechanisms underlying this devastating neurodegenerative disease. Led by pioneering figures such as Beth Stevens, this research emphasizes the pivotal role of microglial cells, which act as the brain’s immune system. These cells are crucial for maintaining brain health by clearing out damaged cells and regulating synapse pruning. However, abnormalities in their function can contribute to Alzheimer’s, Huntington’s, and other neurodegenerative disorders. As the Alzheimer’s Association projects an alarming rise in cases, understanding the connection between microglial activity and disease progression is essential for developing effective treatments and biomarkers that could enhance the lives of millions.
The exploration of Alzheimer’s research encompasses various facets of neurobiology and the immune response within the brain. This vital domain investigates how brain-supporting cells, such as microglia, interact with the neural landscape, influencing cognitive decline and memory loss. By studying neurodegenerative disorders like Alzheimer’s disease, scientists are uncovering pathways that could lead to innovative therapeutic strategies. The insights gained not only deepen our understanding of brain immune mechanisms but also highlight the urgency of addressing the growing challenge posed by aging populations worldwide. With critical contributions from researchers and organizations dedicated to this cause, the fight against these illnesses continues to evolve.
Understanding the Role of Microglial Cells in Alzheimer’s Disease
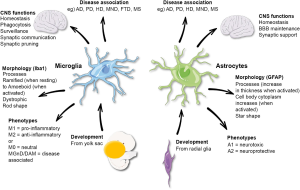
Microglial cells are pivotal players in the brain’s immune system, acting as sentinels to protect neural tissue from damage and disease. This unique cell type continuously surveys the brain environment, identifying and clearing away dead cells and debris caused by injury or neurodegenerative processes. Research led by Beth Stevens has revealed that these cells, while essential for maintaining neuronal health, can also contribute to the progression of Alzheimer’s disease. Aberrations in their normal functions, particularly in synaptic pruning, can exacerbate cognitive decline, highlighting the crucial balance these cells must maintain in brain health.
The connection between microglial activity and neurodegenerative diseases such as Alzheimer’s is becoming increasingly clear. Pathological changes in microglial behavior can lead to chronic inflammation and neuronal loss, hallmark characteristics of Alzheimer’s. This underscores the importance of further investigations into microglial dynamics, not just in Alzheimer’s but across a spectrum of neurodegenerative diseases. With the innovative research emerging from Stevens’ lab, new therapeutic strategies may be developed that target microglial cells, potentially altering the trajectory of these debilitating illnesses.
Innovative Research in Alzheimer’s Disease Treatment
The innovative research conducted by Beth Stevens at the Broad Institute demonstrates a paradigm shift in understanding the immune functions of the brain, particularly in the context of Alzheimer’s disease. By identifying how microglial cells prune neurons, Stevens’ work offers insights into why certain neurodegenerative diseases progress. The discovery that irregular pruning could play a role in Alzheimer’s has opened new avenues for treatment development. As the world faces a growing elderly population, understanding these mechanisms is vital for crafting more effective therapies.
Furthermore, the collaboration with institutions such as the Alzheimer’s Association emphasizes the collective effort required to tackle this widespread disease. These partnerships utilize the latest scientific findings to advocate for early diagnosis and intervention, aiming to provide a better quality of life for the millions affected by Alzheimer’s. The intersection of innovative basic research and practical clinical applications paves the way for potential breakthroughs in how we approach disease management in neurodegenerative disorders.
Funding the Fight Against Alzheimer’s Disease
A significant component of sustaining groundbreaking Alzheimer’s research lies in obtaining adequate funding and support from government agencies and private organizations. Beth Stevens highlights the crucial role that the National Institutes of Health (NIH) and similar institutions have played in advancing her studies on microglial cells and their implications in neurodegenerative diseases. This funding has not only facilitated high-caliber scientific inquiry but has also served as a foundation for developing new therapeutic approaches to combat Alzheimer’s and its related disorders.
The quest for funding is a constant challenge in the field of neuroscience, especially in the realm of Alzheimer’s research, where the potential market for treatments could reach staggering financial sums as the population ages. As projections indicate that the cases of Alzheimer’s could rise significantly, it becomes increasingly evident that proactive investment in scientific research is not just beneficial but necessary. Engaging stakeholders, including philanthropic organizations and public health entities, will be essential in harnessing the resources needed to translate research discoveries into effective treatments.
The Future of Alzheimer’s Research
Alzheimer’s research is entering a transformative phase as new insights about the brain’s immune system and its relationship with neurodegenerative diseases come to light. With experts like Beth Stevens at the forefront, the understanding of microglial functions is set to reshape strategies for diagnosis and treatment. Future research will likely explore how manipulating microglial activity can help restore balance in neuron health, potentially slowing the progression of Alzheimer’s disease and providing new hope to the millions affected.
Additionally, as more discoveries unfold, increased collaboration between academic institutions, healthcare providers, and the private sector will be essential. This integrated approach will facilitate the translation of research findings into clinical practice quicker and more efficiently. Investing in such collaborative frameworks allows for the pooling of expertise and resources, which is crucial for navigating the complex nature of Alzheimer’s and enhancing the quality of life for those impacted by this insidious disease.
Beth Stevens: A Pioneer in Neuroimmune Research
Beth Stevens stands out as a pioneering neuroscientist whose research has illuminated the importance of microglial cells in neurodegenerative diseases, particularly Alzheimer’s. Her work not only identifies the dual role of these cells as protectors and potential aggressors in the brain but also establishes a new understanding of their practical implications in human health. Recognized as a MacArthur genius, Stevens’s contributions continue to drive momentum in this critical field of study, emphasizing the interplay between the brain’s immune response and cognitive function.
Stevens’ innovative approach exemplifies how basic science can yield significant insights into pathology, ultimately leading to new treatment paradigms. As she and her team advance their research, the potential for developing targeted therapies that can mitigate the adverse effects of microglial dysfunction grows. This kind of forward-thinking research is vital to addressing the rising incidence of Alzheimer’s, especially in light of future demographic challenges that will demand new solutions to improve patient outcomes.
Collaboration in Alzheimer’s Research
Collaboration plays a critical role in advancing Alzheimer’s research, as evidenced by the partnerships formed between academic institutions, healthcare organizations, and advocacy groups. By sharing resources, knowledge, and expertise, researchers like Beth Stevens can enhance the understanding of cerebral immunity and its role in Alzheimer’s disease. Such collaborations facilitate multidisciplinary approaches that integrate genetic, cellular, and clinical research for a more holistic understanding of neurodegenerative diseases.
The importance of collaboration and networking cannot be overstated, as the complexities of Alzheimer’s require input from diverse scientific disciplines. Sharing findings and methodologies allows for rapid progress in exploring new therapeutic routes, helping pave the way for innovative solutions. Organizations such as the Alzheimer’s Association actively contribute to these efforts by promoting research funding and providing a platform for scientists to connect with each other and with the broader public to foster awareness, ultimately leading to earlier diagnosis and improved treatment options.
The Intersection of Neuroscience and Medicine
The interdisciplinary nature of Alzheimer’s research highlights the intersection of neuroscience and medicine, particularly in understanding complex brain disorders. Researchers like Beth Stevens are delving into the immune mechanisms at play within the brain, shedding light on how microglial cell functionality can influence not only neurodegeneration but also therapeutic approaches. By integrating findings from molecular biology, genetics, and imaging techniques, the research landscape is becoming increasingly rich and informative.
This interconnectedness is vital for translating laboratory discoveries into clinical practice. As scientists uncover more about the relationship between microglia and Alzheimer’s disease, the potential for developing targeted treatments becomes clearer. Advancements in biomarker identification could lead to earlier detection of Alzheimer’s, ultimately enhancing treatment efficacy. This continuing research connection ensures that progress in one area spurs development and understanding in another, further driving the quest to combat neurodegenerative diseases.
Challenges in Alzheimer’s Disease Research
Despite advancements in Alzheimer’s research, significant challenges remain in understanding and treating this complex disease. One of the main hurdles is the multifactorial nature of Alzheimer’s, where genetic, environmental, and lifestyle factors converge. Researchers like Beth Stevens emphasize the need for comprehensive studies that address these diverse influences on microglial activity and overall brain health. The difficulty in replicating Alzheimer’s disease conditions in laboratory settings makes it paramount for continued investment in innovative methodologies.
Moreover, funding limitations and the need for long-term studies complicate the research landscape, often hindering potential breakthroughs from reaching clinical application. Overcoming these challenges will require a concerted effort from researchers, policymakers, and the public to recognize the urgency of combating Alzheimer’s. Encouraging collaboration and ensuring resources are allocated towards impactful research initiatives will be crucial in improving outcomes for millions afflicted by this disease.
The Importance of Early Detection in Alzheimer’s
Early detection of Alzheimer’s disease is critical for effective management and therapeutic intervention. According to research, including findings influenced by Beth Stevens’s work on microglial cells, identifying biomarkers for Alzheimer’s can lead to timely treatment and may slow disease progression. Awareness around early signs and symptoms is vital, as it enables individuals to seek help sooner, ultimately improving cognitive health outcomes.
As the research community focuses on understanding the underlying mechanisms of Alzheimer’s, fostering public awareness and education about the significance of early diagnosis will become increasingly important. By empowering individuals to recognize the warning signs and encouraging regular cognitive health assessments, the overall quality of life for those at risk can be significantly enhanced. This proactive approach in identifying Alzheimer’s disease illustrates the potential to make substantial strides in combating this pervasive neurodegenerative disorder.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they act as the brain’s immune system, monitoring for signs of illness and injury. Research has shown that dysregulation of these cells can lead to improper pruning of synapses, which is linked to neurodegenerative diseases like Alzheimer’s.
How has Beth Stevens contributed to advancements in Alzheimer’s research?
Beth Stevens has significantly advanced Alzheimer’s research by uncovering how microglial cells interact with neural circuits and contribute to neurodegenerative diseases. Her work highlights the importance of these cells in synaptic pruning and has paved the way for potential new treatments for Alzheimer’s.
What impact does the Alzheimer’s Association predict for the prevalence of Alzheimer’s disease?
According to the Alzheimer’s Association, the number of Americans living with Alzheimer’s could double by 2050, increasing from an estimated 7 million to over 14 million as the population ages. This emphasizes the urgent need for continued Alzheimer’s research and effective care strategies.
Why is basic science important in Alzheimer’s research, according to Beth Stevens?
Basic science is fundamental in Alzheimer’s research as it provides the groundwork for understanding complex mechanisms, like those involving microglial cells. As Beth Stevens emphasizes, curiosity-driven research often leads to unexpected discoveries that can translate into therapies for Alzheimer’s disease.
What are the implications of improper pruning by microglial cells in neurodegenerative diseases?
Improper pruning by microglial cells can exacerbate conditions in neurodegenerative diseases, including Alzheimer’s. This aberrant activity can lead to synaptic loss and neuronal damage, highlighting the need for research to target these processes for potential therapeutic interventions.
How does research on microglial cells aid in the early detection of Alzheimer’s disease?
Research on microglial cells contributes to early detection of Alzheimer’s by identifying biomarkers linked to their activity and the pathological processes they regulate. Recognizing these biomarkers can help diagnose Alzheimer’s disease at an earlier stage, improving outcomes for patients.
What funding sources support Alzheimer’s research, particularly in relation to microglial studies?
Alzheimer’s research, particularly studies on microglial cells, is often supported by funding from federal agencies, including the National Institutes of Health (NIH). This funding is crucial for carrying out basic science research that can lead to breakthroughs in understanding and treating Alzheimer’s.
| Key Points |
|---|
| Beth Stevens is a neuroscientist leading research on microglial cells, crucial for brain immune functions. |
| Microglia help maintain brain health by clearing damaged cells and pruning synapses, but can contribute to diseases like Alzheimer’s when faulty. |
| Stevens’ lab shows that improper pruning relates to Alzheimer’s and other neurodegenerative disorders, paving the way for new treatments. |
| Research backed by federal funding, mainly from the NIH, is critical for advancing our understanding and treatment of Alzheimer’s disease. |
| The potential for early detection of Alzheimer’s is enhanced by new biomarkers resulting from this research. |
| With the aging U.S. population, the number of Alzheimer’s cases may double by 2050, emphasizing the urgency for more effective research and treatment. |
Summary
Alzheimer’s research is at the forefront of understanding and combating one of the most pressing health challenges of our time. With significant contributions from researchers like Beth Stevens, who emphasizes the role of microglial cells, our depiction of brain immunity and its disorders is evolving. The continued exploration of these immune responses offers promising avenues for treatment and early detection, critical as we brace for a potential surge in Alzheimer’s cases in the coming decades. Stevens’ commitment to curiosity-driven science reinforces that foundational research is essential for unlocking the mysteries of neurodegenerative diseases.