Microglial research is at the forefront of understanding the brain’s immune system, particularly in relation to neurodegenerative diseases like Alzheimer’s disease. These remarkable cells, known as microglia, play a crucial role in maintaining brain health by pruning synapses and eliminating dead or damaged neurons. Unfortunately, when this delicate balance is disrupted, it can contribute to the progression of harmful conditions such as Alzheimer’s and Huntington’s diseases. Researchers, notably Beth Stevens at Boston Children’s Hospital, are uncovering how improper synaptic pruning by microglia may lead to significant cognitive decline in affected individuals. This ground-breaking research not only aims to unveil new biomarkers but also enhances our ability to develop targeted drugs for treating neurodegenerative diseases, potentially transforming the lives of millions.
Exploring the landscape of microglial cells reveals their vital function in the brain’s defensive mechanisms against various neurodegenerative disorders, such as Alzheimer’s and Huntington’s diseases. Often referred to as the brain’s immune cells, these microglia are essential for maintaining synaptic integrity through their process of synaptic refinement. However, aberrations in their activity can lead to detrimental outcomes, influencing the onset and progression of cognitive impairments. Researchers, including prominent figures like Beth Stevens, are dedicated to advancing our understanding of these critical brain components. Their innovative studies are paving the way for promising new therapies and diagnostic tools that target the underlying causes of these devastating diseases.
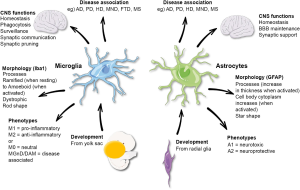
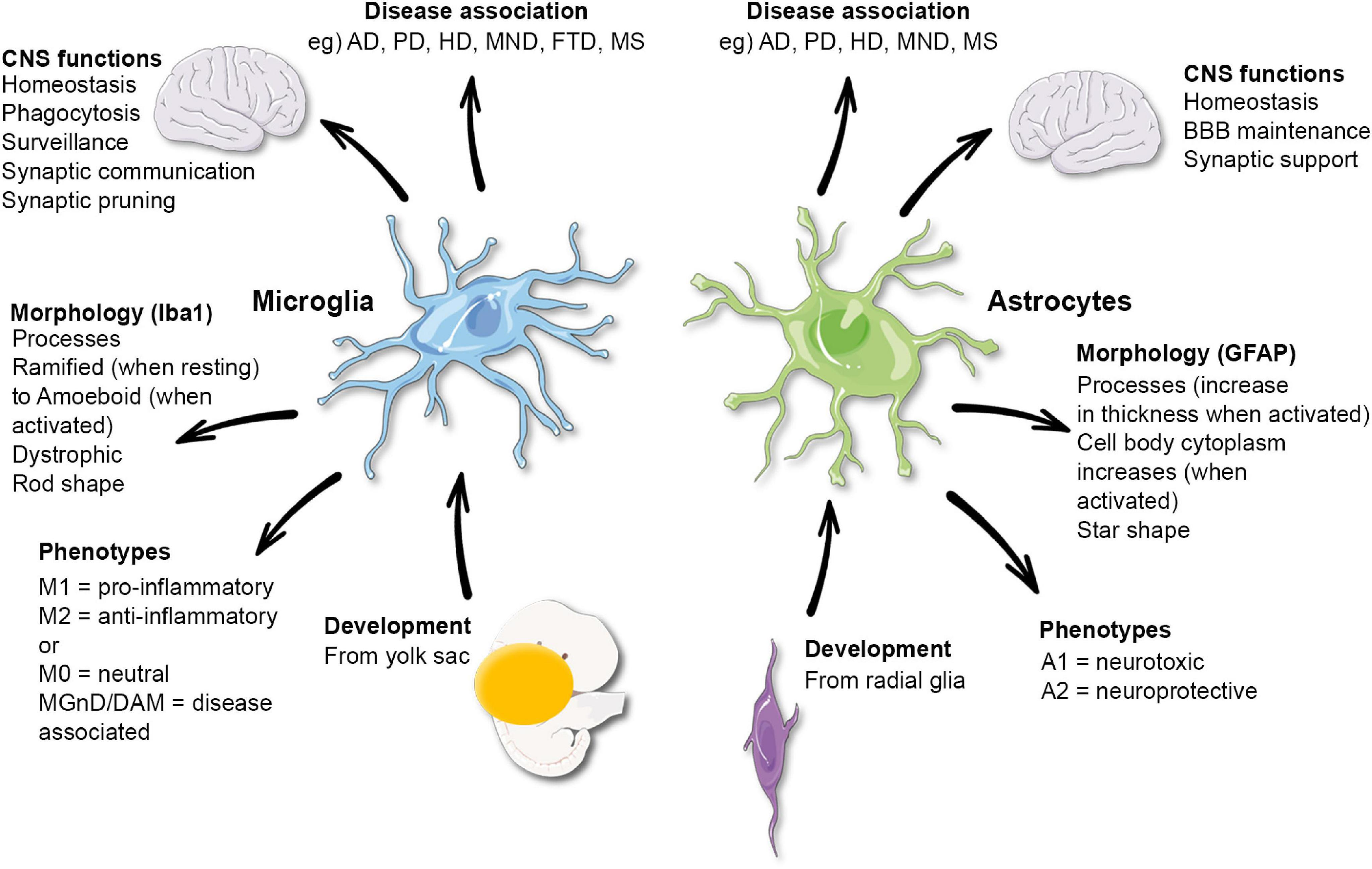
Understanding Microglia and Their Role in the Brain
Microglia are a crucial component of the brain’s immune system, acting as the first line of defense against injury and disease. These specialized cells constantly monitor the brain environment for signs of trouble, playing a pivotal role in maintaining neural health. Their functions include the removal of dead or damaged neurons and the regulation of synaptic pruning – a process essential for healthy brain development and function. However, an imbalance in microglial activity can lead to detrimental effects, contributing to conditions such as Alzheimer’s disease. Understanding the dual nature of microglia, both protective and potentially harmful, is vital to advancing therapeutic strategies against various neurodegenerative diseases.
Research into microglia has highlighted their involvement in not only normal brain function but also in the pathogenesis of neurodegenerative diseases. For instance, in Alzheimer’s disease, microglia can become overactive, leading to excessive synaptic pruning that disrupts communication between neurons. This aberration can accelerate cognitive decline as seen in Alzheimer’s patients. Furthermore, by exploring how microglial activation patterns differ between healthy brains and those affected by neurodegeneration, scientists can establish biomarkers that might help in diagnosing Alzheimer’s earlier, potentially improving outcomes for millions of individuals.
The Importance of Synaptic Pruning in Neurodegenerative Diseases
Synaptic pruning is a vital process through which the brain refines its neural connections. During development, microglia assist in removing excess synapses, ensuring that only the most efficient pathways remain. This selective pruning is critical not only for normal development but also for the ongoing adaptability of the brain in response to learning and experiencing, underscoring the importance of synaptic health in cognitive functions. However, in the context of neurodegenerative diseases like Alzheimer’s, the mechanisms governing synaptic pruning can malfunction, leading to the loss of synaptic connections that are crucial for memory and cognition.
Emerging studies indicate that abnormal synaptic pruning, driven by microglial dysfunction, may be a key factor in the progression of neurodegenerative diseases. Researchers are now focused on identifying how disruptions in this process can lead to clinical symptoms associated with Alzheimer’s and similar disorders. By understanding the fine balance between adequate synaptic elimination and excessive loss, scientists can work toward developing therapeutic interventions that restore proper synaptic pruning, potentially altering the course of diseases like Alzheimer’s.
Innovations in Microglial Research
Recent advancements in microglial research have provided new insights into the role of these cells in neurodegenerative diseases. Investigators like Beth Stevens are harnessing innovative techniques to explore the molecular pathways that govern microglial behavior. Their findings have illuminated how microglial activation states can influence neuronal health and contribute to synaptic dysfunction in conditions such as Alzheimer’s disease. By identifying specific signaling pathways and molecular markers associated with microglial activity, researchers are laying the groundwork for new therapeutic strategies aimed at modulating these immune cells to better support brain health.
Moreover, the application of cutting-edge technologies, including gene editing and advanced imaging techniques, is revolutionizing microglial research. These tools allow scientists to observe and manipulate microglial functions in real-time, providing unprecedented access to the dynamics of the brain’s immune response. As researchers continue to unravel the complexities of microglia, there is a growing optimism that this work will lead to identifying novel therapeutic targets for Alzheimer’s disease and other neurodegenerative disorders, ultimately providing hope for those affected.
The Impact of Federal Funding on Neuroscience Research
The role of federal funding in advancing neuroscience research cannot be overstated, particularly in understanding complex conditions like Alzheimer’s disease. As highlighted by Beth Stevens, much of her lab’s innovative work on microglial function and synaptic pruning was made possible by timely support from agencies like the National Institutes of Health (NIH). This foundational funding is crucial for researchers, enabling them to delve into basic science that may not yield immediate results but is essential for uncovering the underlying mechanisms of neurodegenerative diseases that impact millions of lives.
In the realm of scientific research, many groundbreaking discoveries often emerge from seemingly unrelated studies. Stevens emphasizes that the exploration of subjects, such as how a mouse perceives its environment, can ultimately inform our understanding of human health. The long-term vision supported by federal funding allows researchers to pursue high-risk, high-reward projects that could lead to significant advancements in treatments and therapies for diseases such as Alzheimer’s. The continuity of support is vital for fostering curiosity and innovation within the scientific community.
The Future of Alzheimer’s Disease Research
As the number of individuals affected by Alzheimer’s disease continues to rise, there is an urgent need for innovative research to uncover effective treatments. Current research efforts are increasingly focusing on the intersection of neurodegenerative diseases and the immune system, particularly the role of microglia in maintaining brain homeostasis. By understanding how microglial dysfunction contributes to the progression of Alzheimer’s, researchers aim to identify potential intervention points where therapies can be applied to slow or halt disease progression. This shift in focus holds promise for developing targeted treatments that are more effective and have fewer side effects.
Looking forward, interdisciplinary collaboration will be essential in addressing the complexities of Alzheimer’s disease. By bringing together neuroscientists, immunologists, geneticists, and pharmaceutical scientists, a more comprehensive understanding of the disease can be achieved. Collaborative research may lead to the development of innovative biomarkers for early detection and the creation of novel therapeutic approaches that utilize the body’s own immune responses. With continued dedication and investment in research, the outlook for Alzheimer’s disease may become significantly more hopeful in the coming years.
Recent Advances in Biomarker Development
The development of biomarkers for Alzheimer’s disease represents one of the most promising areas of research in neuroscience today. Biomarkers are measurable indicators of a biological state that can provide insight into disease onset, progression, and treatment responses. Recent advances have highlighted the potential of using microglial activity as a biomarker to assess inflammation in the brain and its correlation with cognitive decline in Alzheimer’s disease. By monitoring changes in microglial behavior, researchers can gain valuable insights into disease progression and response to therapeutic interventions.
Moreover, advancements in neuroimaging techniques have enabled scientists to visualize microglial activation in living patients, paving the way for longitudinal studies that could better predict disease trajectories. The emergence of new blood-based biomarkers is also under investigation, which might offer a non-invasive method for monitoring brain health in individuals at risk for developing Alzheimer’s. Together, these innovations are setting the stage for a future where early diagnosis and tailored treatments could significantly enhance the management of Alzheimer’s disease.
The Role of Synaptic Dysfunction in Alzheimer’s Pathogenesis
Synaptic dysfunction is increasingly recognized as a central feature of Alzheimer’s disease pathology. In healthy brains, microglial cells play an essential role in the elimination of excess synapses during development, ensuring efficient neural signaling. However, in Alzheimer’s, the balance of synaptic pruning becomes compromised, leading to synaptic loss and, ultimately, cognitive deficits. Understanding the mechanisms that drive this dysfunction not only helps clarify the etiology of Alzheimer’s but also aids in identifying potential therapeutic targets.
Investigating the interaction between microglia and neurons during synaptic pruning has opened new avenues for Alzheimer’s research. It is becoming evident that disrupted communication between these cell types can exacerbate the disease’s symptoms. By exploring the pathways involved in this communication, scientists are developing a new understanding of how to prevent synaptic loss and promote cognitive resilience. The potential to modulate these interactions offers hope for developing effective treatment modalities aimed at preserving synaptic integrity in Alzheimer’s disease.
Challenges in Alzheimer’s Disease Research
Despite significant progress in understanding the biology of Alzheimer’s disease, numerous challenges remain in the field of research. One of the main obstacles is the complexity and heterogeneity of the disease itself, which can manifest differently in individuals. This variability makes it difficult to identify universal biomarkers and develop treatments that would be effective across the entire patient population. Additionally, the aging population poses challenges for researchers, as age is the most significant risk factor for Alzheimer’s, making it imperative to refine research strategies that can keep pace with demographic changes.
Another critical challenge lies in the translation of basic scientific discoveries into clinical applications. Although fundamental research, such as that conducted in the Stevens Lab on microglial function and synaptic pruning, lays the groundwork for new therapeutic approaches, moving from laboratory findings to effective treatments is often fraught with hurdles. Bridging the gap between discovery and implementation requires collaboration across disciplines and persistence in seeking new funding opportunities to support innovative research endeavors.
The Interplay Between Neuroinflammation and Neurodegeneration
Neuroinflammation has emerged as a significant component in the progression of neurodegenerative diseases like Alzheimer’s. Microglia, as primary immune cells in the brain, play a pivotal role in mediating inflammatory responses to cellular damage or amyloid plaques accumulation. While their activation is critical for initiating protective responses, chronic activation can lead to neuroinflammation that exacerbates neuronal loss and synaptic dysfunction. Understanding the delicate interplay between neuroinflammation and neurodegeneration is key to unraveling the complexities of diseases such as Alzheimer’s.
Current research efforts aim to dissect the molecular pathways that regulate microglial activation and their contribution to neuroinflammation. By exploring these pathways, scientists hope to identify novel therapeutic targets that could mitigate the harmful effects of chronic inflammation on brain health. Ultimately, developing treatments that balance the protective and harmful aspects of neuroinflammation could alter the trajectory of Alzheimer’s disease, leading to improved outcomes for those at risk.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial in Alzheimer’s disease research as they constitute the brain’s immune system. They monitor the brain for signs of damage and remove dead cells while performing synaptic pruning, a process that helps maintain healthy neuronal communication. However, improper microglial activity can lead to neurodegenerative diseases like Alzheimer’s, making them a significant focus of current studies aimed at understanding disease mechanisms and developing potential therapies.
How does synaptic pruning by microglia affect neurodegenerative diseases?
Synaptic pruning by microglia is essential for normal brain function and development, but aberrant pruning can contribute to neurodegenerative diseases like Alzheimer’s. Research indicates that when microglia fail to regulate synaptic connections properly, it can lead to cognitive decline and neuronal loss, highlighting the importance of understanding microglial behavior in the context of neurodegenerative conditions.
What findings have been made regarding microglia and the brain’s immune system?
Recent findings in microglial research have revealed that these cells are integral to the brain’s immune system, actively participating in the removal of dysfunctional cells and modulating synaptic connections. Studies led by researchers like Beth Stevens have shown that microglia not only respond to injury but can also adversely affect neuronal health in diseases like Alzheimer’s, indicating their dual role in both protection and risk.
How does microglial research contribute to potential Alzheimer’s treatments?
Microglial research is paving the way for new Alzheimer’s treatments by identifying markers and mechanisms by which these immune cells contribute to disease progression. Understanding the roles of microglia in synaptic pruning and inflammation enables researchers to target them with therapies aimed at correcting improper microglial behavior, potentially slowing or halting neurodegeneration observed in Alzheimer’s patients.
What foundational research has influenced current microglial studies related to Alzheimer’s disease?
Current microglial studies related to Alzheimer’s disease have been significantly influenced by foundational research supported by institutions like the NIH. Pioneering work in understanding how microglia interact with neurons has laid the groundwork for exploring their implications in neurodegenerative diseases. Scientists have leveraged insights from early curiosity-driven studies to investigate how microglial dysfunction leads to conditions such as Alzheimer’s, thus shaping future therapeutic strategies.
What are some challenges in microglial research related to Alzheimer’s disease?
Challenges in microglial research related to Alzheimer’s disease include the complexity of microglial functions and their dual roles in health and disease. Research must unravel the specific conditions under which microglia shift from protective to detrimental actions. Additionally, translating findings from animal models to humans poses its own difficulties, as the understanding of microglial behavior must account for species differences and disease heterogeneity.
| Key Points |
|---|
| Neuroscientist Beth Stevens is at the forefront of research on microglial cells and their role in neurodegenerative diseases like Alzheimer’s. |
| Microglia serve as the brain’s immune system, helping to remove damaged cells and prune synapses necessary for communication between neurons. |
| Improper pruning by microglia has been linked to Alzheimer’s and Huntington’s diseases, highlighting the need for ongoing research in this area. |
| Stevens’ lab at Boston Children’s Hospital focuses on understanding these processes to develop new biomarkers and treatments for neurodegenerative disorders. |
| Funding from federal agencies, especially the NIH, has been crucial in supporting Stevens’ research and its trajectory over the years. |
| Basic science research, though it may initially appear unrelated to practical applications, is vital for unlocking mysteries related to diseases. |
Summary
Microglial research has become a vital area of focus in understanding and combating neurodegenerative diseases. The groundbreaking work led by Beth Stevens has revealed the critical balance between microglial function and disease pathology, particularly in Alzheimer’s disease. By examining how microglial cells contribute to synaptic pruning and immune response in the brain, researchers are uncovering new pathways that could lead to innovative biomarkers and treatments. The foundation of this research underscores the importance of curiosity-driven science supported by robust federal funding, paving the way for advances that may one day improve the lives of millions affected by Alzheimer’s.